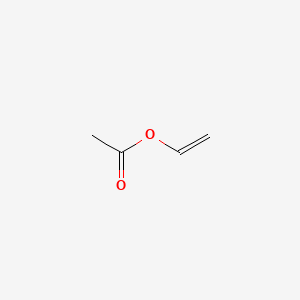
Vinyl acetate ch 2 cho 2 cch 3 is prepared from ethylene by reaction with oxygen and acetic acid over a palladium catalyst under the action of free radical initiators vinyl acetate monomers single unit molecules can be linked into long branched polymers large multiple unit molecules in which the structure of the vinyl acetate repeating units is.
Weight fraction of a poly vinyl acetate.
1 the solubilities increased linearly with increasing pressure and decreased with increasing temperature.
Since vinyl alcohol is highly unstable with respect to acetaldehyde the preparation of vinyl acetate is more complex than the synthesis of other acetate esters.
Many polymeric materials having chain like structures similar to polyethylene are known.
Red hot white vinyl adhesive glue 2 pack 1 5 ounce tube 4 2 out of 5 stars 5 9.
Control polymers possessed of no.
3 the monomer vinyl acetate was first produced on an industrial scale by the addition of acetic acid to acetylene with a mercury i salt 4 but it is now primarily made by palladium catalyzed oxidative addition of acetic acid to ethylene.
While the major industrial applications of vinyl acetate produce copolymers with such monomers as vinyl chloride or ethylene substantial amounts of the homopolymers of vinyl acetate are also used.
Sodium acetate trihydrate hot ice 99 6 min.
1 and table 3 correlation results of the sl eos are shown in fig.
Solubilities of ethylene in molten eva were measured at temperatures of 393 15 k 443 15 k and 473 15 k and at pressures up to 10 mpa.
Vinyl acetate is the acetate ester of vinyl alcohol.
Experimental results are shown in fig.
Burndy pen a13 8 oxide inhibiting joint compounds penetrox a 13 8 oz container size squeeze bottle container type silver.
Polymers formed by a straightforward linking together of monomer units with no loss or gain of material are called addition polymers or chain growth polymers a listing of some important addition polymers and their monomer precursors is presented in the following table.
The major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst.
Poly vinyl acetate was discovered in germany in 1912 by fritz klatte.
Poly vinyl acetate containing a small amount of n benzyl 4 vinylpyridinium chloride pvac co vpc and that containing 16 mol of methyl acrylate and a small amount of the pyridinium group pvac co ma co vpc showed significant degradation when placed in an aeration tank of sewage works.